
电话:+86-755-86967710
邮箱:webmaster@szbl.ac.cn

Institute of Infectious Diseases
chenmx(at)szbl.ac.cn
Pathogenic Infection and Immune Responses,Immune Responses and Metabolism,Immunology,Microbiology
Junior Principal Investigator
Postdoctoral Fellow
PhD
Bachelor
Exploring Biological Targets for the Prevention and Treatment of Infectious Diseases. Our research group primarily investigates the pathogenic mechanisms of pathogens and the molecular mechanisms by which the host immune system and cellular metabolism defend against these pathogens. This includes: 1. The pathogenic mechanisms of microorganisms; 2. The molecular mechanisms of the body’s resistance to pathogen infection; 3. The screening and identification of new antimicrobial effectors. Our research group has a strong academic atmosphere, with harmonious and supportive members who strive for progress together. We welcome students or researchers interested in the fields of infection, immunity, metabolism, and related studies to join our group.
Dr. Meixin Chen completed her Ph.D. under the supervision of Professor Jun Cui at Sun Yat-sen University in 2018. She then joined the research group of Dr. Jorge E. Galán, a pioneer in pathogenic infection, at Yale University School of Medicine for postdoctoral studies, where her primary focus was on immunometabolism and immune regulation in infection. In 2021, she joined the Shenzhen Bay Laboratory as a distinguished researcher at the Institute of Infectious Diseases. Dr. Chen has long been dedicated to studying host immune responses and pathogen interactions. Her research was the first to reveal the critical function and regulatory mechanisms of cGAS protein ubiquitination, a DNA sensor, in defending against pathogenic infections. She also pioneered the use of metabolite sensors to uncover the antimicrobial pathways of the metabolite itaconate in host cells. Dr. Chen’s work sheds light on the mechanisms of pathogen virulence and host defense, contributing to the broader knowledge of host-pathogen interactions. Her findings have been published in Science, Molecular Cell, Nature Microbiology, Advanced Science, and Nature Communications. As an independent corresponding author, she has submitted significant work to Nature Metabolism (revised and resubmitted) and Nature Microbiology (submitted). Her team applied three patents, and she has led projects funded by the National Natural Science Foundation, Shenzhen Medical School Special Fund, among others. Dr. Chen has received accolades including the Guangdong Pearl River Talent Program for Young Talents and Shenzhen National A-Level Leading Talent recognition.
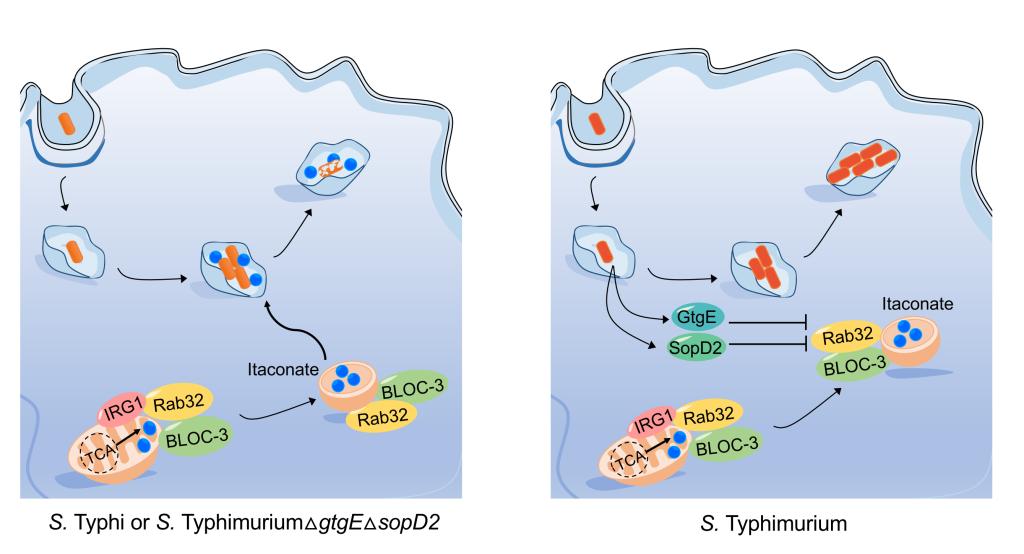
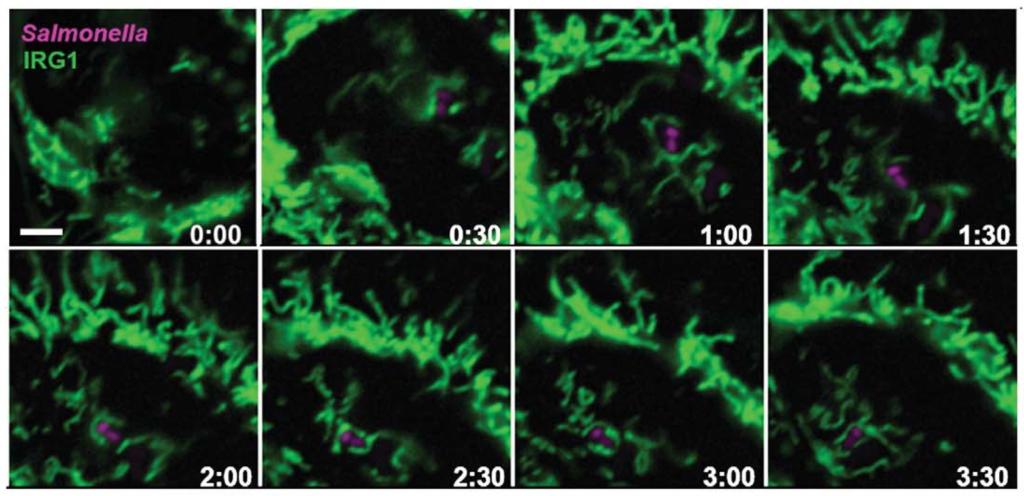
the antibacterial pathway of mitochondrial itaconate
Meixin Chen, et al. Science 369.6502 (2020): 450-455.
1.Chen, M.#, Sun, H. #, Boot, M., Shao, L., Chang, S.J., Wang, W., Lam, T.T., Lara-Tejero, M., Rego, E.H. and Galán, J.E. *, 2020. Itaconate is an effector of a Rab GTPase cell-autonomous host defense pathway against Salmonella. Science, 369(6502), pp.450-455.
2.Lian, H. #, Park, D. #,Chen, M., Schueder, F., Lara-Tejero, M., Liu, J., & Galán, J. E*, 2023. Parkinson’s disease kinase LRRK2 coordinates a cell-intrinsic itaconate-dependent defence pathway against intracellular Salmonella. Nature Microbiology, 1-16.
These pubilications demonstrate that Rab32/LRRK2 facilitates the delivery of itaconate to the Salmonella-containing vacuoles, which allows itaconate to inhibit bacterial growth. Our results have been cited by the itaconate expert Luke O’Neil for several times in articles/reviews. The HHMI professor John D. MacMicking cited our publication in their Science paper revealing the important antibacterial protein APOL3. Karine Auclairp group has repeated our key results successfully and made some other discoveries.
3.Chen, M.#, Zhao, Z. #, Meng, Q. #, Liang, P. #, Su, Z., Wu, Y., Huang, J. and Cui, J. *, 2019. TRIM14 Promotes Noncanonical NF‐kB Activation by Modulating p100/p52 Stability via Selective Autophagy. Advanced Science, p.1901261.
This paper demonstrates that TRIM14 enhances p100/p52 processing in Noncanonical NF-kB signaling. It has been cited by many groups. Jiankun Wu group repeated our key results and made some other discoveries.
4.Chen, M.#, Meng, Q. #, Qin, Y. #, Liang, P., Tan, P., He, L., Zhou, Y., Chen, Y., Huang, J. *, Wang, R.F. * and Cui, J. *, 2016. TRIM14 inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Molecular cell, 64(1), pp.105-119.
This paper firstly demonstrated the mechanical details of cGAS ubiquitination, which was important in autophagic degradation of cGAS and resisting virus infection in innate immunity. It has been cited by many groups in their research articles or reviews, including Katherine A. Fitzgerald and Beth Levine, who are experts in the field of innate immunity and autophagy.
Contact
Address: Gaoke Innovation Center,Guangming District, Shenzhen
Phone: +86-755-86967710
Email: webmaster@szbl.ac.cn
Postal Code: 518132
Copyright © 2025 Shenzhen Bay Laboratory. All Rights Reserved.